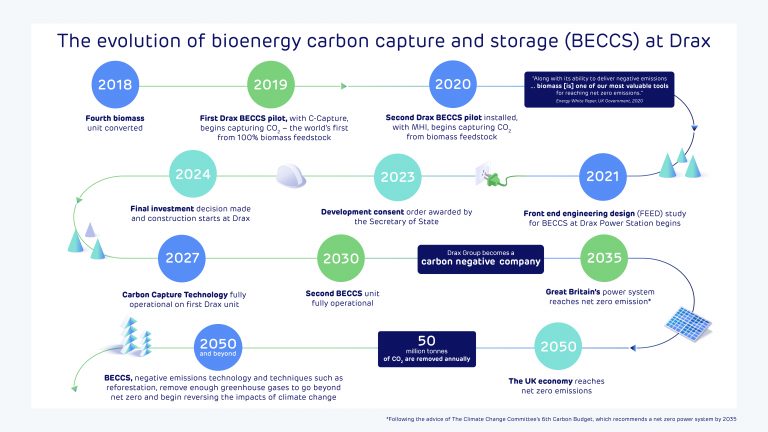
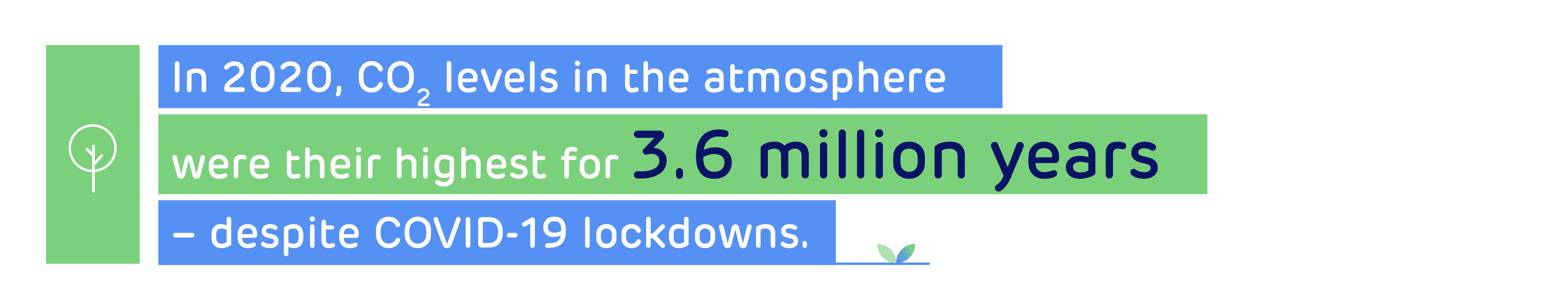
Richard Gwilliam, Head of Cluster Development at Drax
Key takeaways:
- The Humber industrial cluster contributes £18 billion a year to the UK economy and supports 360,000 jobs in heavy industry and manufacturing.
- As demand for industrial products with green credentials rises and net zero targets demand decarbonisation, businesses in the Humber need to begin implementing carbon capture at scale.
- The size of the Humber and diversity of industries make it a significant challenge but if we get it right, the Humber will be a world leader in decarbonisation.
- Without investment in decarbonisation infrastructure the region risks losing its status as a world leading industrial cluster putting hundreds of thousands of jobs at risk.
When the iconic Humber Bridge opened in June 1981, it did more than just set records for its size. It connected the region, uniting both communities and industries, and allowing the Humber to become what it is today: a thriving industrial hub that contributes more than £18 billion to the UK economy and supports some 360,000 jobs.
As the UK works towards a low-carbon future, the shift to a green economy will require new regional infrastructure, that once again unites the Humber’s people and businesses around a shared goal.
While the Humber Bridge connected the region across the estuary waters, a new subterranean pipeline that can transport the carbon captured from industries, will unify the region’s decarbonisation efforts.
It’s infrastructure that will be crucial in helping the UK reach its net zero goals, but also cement the Humber’s position as a global decarbonisation leader.

The Humber Bridge
Capturing carbon across the Humber
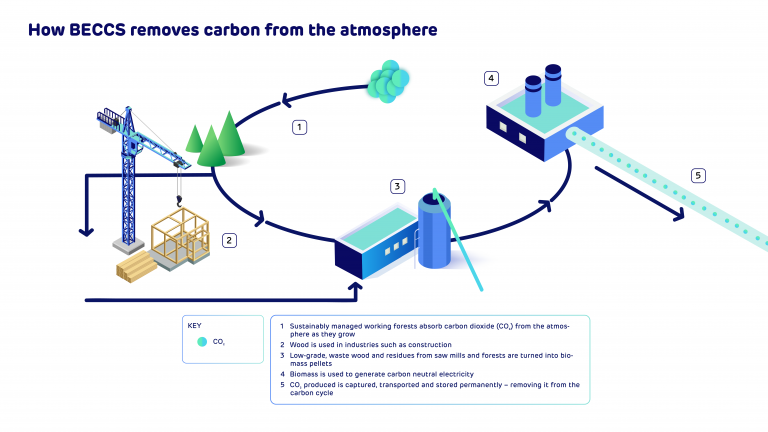
Capturing carbon, preventing emissions from entering the atmosphere and storing them safely and permanently, is a fundamental part of decarbonising the economy and tackling climate change. Aside from the chemical engineering required to extract carbon dioxide (CO2) from industrial emissions, one of the key challenges of carbon capture is how you transport it at scale to secure storage locations, such as below the North Sea bed where the carbon can be permanently trapped and sequestered.

Engineers at Drax Power Station
At Drax, we’re pioneering bioenergy with carbon capture and storage (BECCS) technology. But carbon capture will play an important role in decarbonising a wide range of industries. The Humber region not only produces about 20% of the UK’s electricity, it’s also a major hub for chemicals, refining, steel making and other carbon-intensive industries.
The consequence of this industrial mix is that the Humber’s carbon footprint per head of population is bigger than anywhere else in the country. At an international level it’s the second largest industrial cluster by CO2 emissions in the whole of Western Europe. If the UK is to reach net zero, the Humber must decarbonise. And carbon capture and storage will be instrumental in achieving that.
The scale of the challenge in the Humber also makes it an opportunity to significantly reduce the country’s overall emissions and break new ground, implementing carbon capture innovations across a wide range of industries. These diverse businesses can be united in their collective efforts and connected through shared decarbonisation infrastructure – equipment to capture emissions, pipelines to transport them, and a shared site to store them safely and permanently.
Economies of scale through shared infrastructure
The idea of a CO2 transport pipeline traversing the Humber might sound unusual, but large-scale natural gas pipelines have criss-crossed the region since the late 1960s when gas was dispatched from the Easington Terminal on the east Yorkshire coast under the Humber to Killingholme in North Lincolnshire. Further, the UK’s existing legislation creates an environment to ensure they can be operated safely and effectively. CO2 is a very stable molecule, compared to natural gas, and there are already thousands of miles of CO2 pipelines operating around the US, where it’s historically been used in oil recovery.
A shared pipeline also offers economies of scale for companies to implement carbon capture, allowing the Humber’s cluster of carbon-intensive industries to invest in vital infrastructure in a cost-effective way. The diversity of different industries in the region, from renewable baseload power generation at Drax to cutting-edge hydrogen production, also offers a chance to experiment and showcase what’s possible at scale.
The Humber’s position as an estuary onto the North Sea is also advantageous. Its expansive layers of porous sandstone offer an estimated 70 billion tonnes of potential CO2 storage space.

The Humber Estuary
But this isn’t just an opportunity to decarbonise the UK’s most emissions-intensive region, it’s a stage to present a new green industrial hub to the world. A hub that could create as many as 47,800 jobs, including high quality technical and construction roles, as well as other jobs throughout supply chains and the wider UK economy.
British innovation as a global export
As industries of all kinds across the world race to decarbonise, there’s an increasing demand for products with green credentials. If we can decarbonise products from the region, such as steel, it will give UK businesses a global edge. Failure to follow through on environmental ambitions, however, will not just damage the cluster’s status, it will put hundreds of thousands of jobs at risk.
Breaking new ground is difficult but there are first-mover advantages. The products and processes trialled and run at scale within the Humber offer intellectual property that industrial hubs around the world are searching for, creating a new export for the UK.
But this vision of a decarbonised Humber, that exports both its products and knowledge to the world, is only possible if we take the right action now. We have a genuine global leadership position. If we don’t act now, that will be lost.
Through projects like Zero Carbon Humber and the East Coast Cluster, alongside Net Zero Teesside, the region’s businesses have shown our collective commitment to implementing decarbonisation at scale through collaboration.
As a Track 1 cluster, the Humber presents one of the UK’s greatest opportunities to level up – attracting global businesses and investors, as well as protecting and creating skilled jobs. We need to seize this moment and put in place the infrastructure that will put the Humber at the forefront of a low-carbon future.











 Scope Two emissions are those which come from the generation of energy an organisation uses. These can include emissions form electricity, steam, heating, and cooling.
Scope Two emissions are those which come from the generation of energy an organisation uses. These can include emissions form electricity, steam, heating, and cooling.